|
|
| ・ |
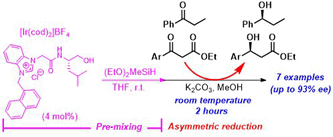
T. Matsuki, H. Teramoto, R. Ichihara, K. Inui, S. Sakaguchi, Asymmetric Silane Reduction of Ketones and b-Keto Esters Catalyzed by a
Chiral Azolium/Iridium System in the Presence of a Base in Methanol at
Room Temperature, Results in Chemistry, 4, 100364 [7 papes] (2022). |
|
 |
|
|
| ・ |
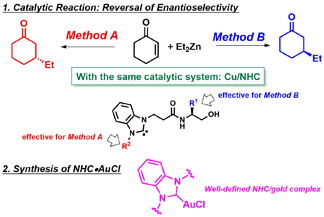
Y. Nakano, S. Shimizu, C. Takeda, S. Sakaguchi, Reversal of Enantioselectivity in the Conjugate Addition Reaction of Cyclic
Enones with the CuOTf/AzoliumCatalytic System, Molecules, 26, 3404 [12 papes] (2021). |
|
 |
|
|
| ・ |
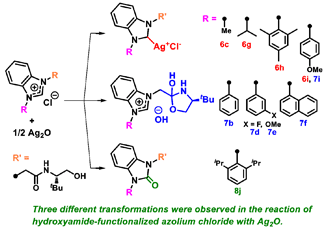
K. Kamiguchi, S. Sakaguchi, Reaction of hydroxyamide-functionalized azolium salt with Ag2O: Three different transformations, J. Organomet. Chem., 929, 121556 [7 pages] (2020). |
|
 |
|
|
| ・ |
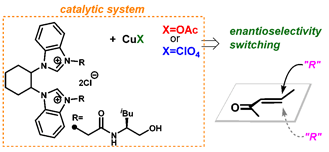
A. Ishibashi, S. Kamihigashi, Y. Iwai, S. Sakaguchi, (±)-trans-1,2-Cyclohexanediamine-Based Bis(NHC) Ligand for Cu-Catalyzed
Asymmetric Conjugate Addition Reaction, Catalysts, 9, 780 [21 pages] (2019). |
|
 |
|
|
| ・ |
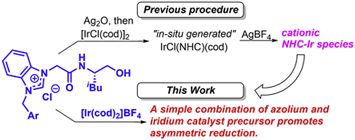
H. Teramoto, S. Sakaguchi, Enantioselective catalytic hydrosilylation of
propiophenone with a simple combination of a cationic iridium complex and
a chiral azolium salt, J. Organomet. Chem., 875, 52-58 (2018). |
|
 |
|
|
| ・ |
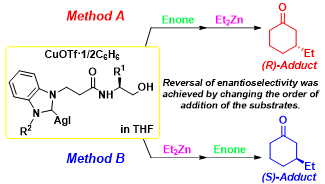
Y. Nakano, S. Sakaguchi, Inversions in asymmetric conjugate addition reaction
of cyclic enones catalyzed by the Cu/NHC-AgX system: Factors affecting
the stereoselective formation of both enantiomers, J. Organomet. Chem., 846, 407-416 (2017). |
|
 |
|
|
| ・ |
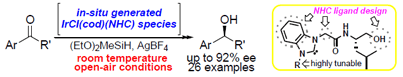
Y. Manabe, K. Shinohara, H. Nakamura, H. Teramoto, S. Sakaguchi, Chiral
N-heterocyclic carbene iridium catalyst for the enantioselective hydrosilane
reduction of ketones, J. Mol. Cat. A: Chem., 421, 138-145 (2016). |
|
 |
|
|
| ・ |
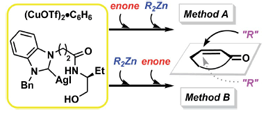
K. Matsumoto, Y. Nakano, N. Shibata, S. Sakaguchi, Enantioselectivity switch
in copper-catalyzed conjugate addition reactions under the influence of
a chiral N-heterocyclic carbene-silver complex, RCS Adv., 6, 7755-7759 (2016). |
|
 |
|
|
| ・ |
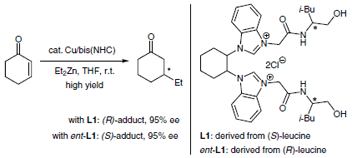
S. Kamihigashi, N. Shibata, S. Sakaguchi, Cu-catalyzed asymmetric conjugate
addition of dialkylzincs to enones using (±)-trans-1,2-cyclohexanediamine-based
bis(NHC) derived from L-leucinol, Synlett, 25, 2933-2937 (2014). |
|
 |
|
|
| ・ |
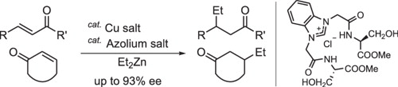
J. Kondo, A. Harano, K. Dohi, S. Sakaguchi, C2-symmetric functionalized azolium salt from serine ester for Cu-catalyzed
asymmetric conjugate addition reaction, J. Mol. Cat. A: Chem., 395, 66-71 (2014). |
|
 |
|
|
| ・ |
K. Shinohara, S. Kawabata, H. Nakamura, Y. Manabe, S. Sakaguchi, Enantioselective
Hydrosilylation of Ketones Catalyzed by a Readily Accessible N-Heterocyclic
Carbene-Ir Complex at Room Temperature, Eur. J. Org. Chem., 2014(25), 5532-5539 (2014). |
|
 |
|
|
| ・ |
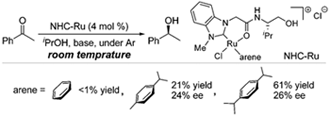
M. Yoshimura, R. Kamisue, S. Sakaguchi, Synthesis of Ru(II) complexes containing
N-heterocyclic carbenes functionalized with secondary donor groups: Catalytic
activity towards enantioselective transfer hydrogenation, J. Organomet. Chem., 740, 26-32 (2013). |
|
 |
|
|
| ・ |
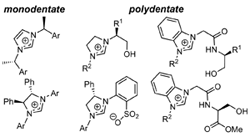
坂口 聡,キラルなアゾリウム塩による銅触媒不斉共役付加反応,有合化,71(4),319-329 (2013). [invited review] |
|
 |
|
|
| ・ |
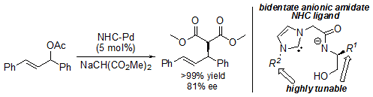
H. Shirasaki, M. Kawakami, H. Yamada, R. Arakawa, S. Sakaguchi, Highly tunable anionic tethered N-heterocyclic carbene of Pd(II) complexes
for asymmetric allylic alkylation reaction, J. Organomet. Chem., 726, 46-55 (2013). |
|
 |
|
|
| ・ |
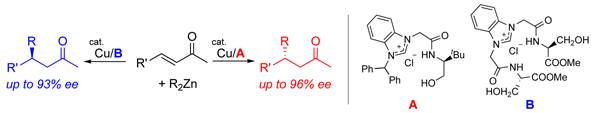
K. Dohi, J. Kondo, H. Yamada, R. Arakawa, S. Sakaguchi, Functionalized N-Heterocyclic Carbene Ligands for Dual Enantioselective Control in the Cu-Catalyzed Conjugate Addition of Dialkylzinc Compounds to Acyclic Enones, Eur. J. Org. Chem., 2012(36), 7143-7152 (2012). [Synfacts 2013, 9, 183.] |
|
 |
|
|
| ・ |
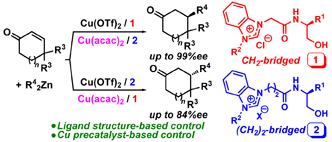
N. Shibata, M. Yoshimura, H. Yamada, R. Arakawa, S. Sakaguchi, Hydroxy-amide-functionalized
Azolium Salts for Cu-catalyzed Asymmetric Conjugate Addition: Stereocontrol Based on Ligand Structure and Copper Precatalyst, J. Org. Chem., 77(8), 4079-4086 (2012). |
|
 |
|
|
| ・ |
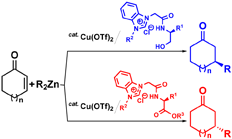
M. Yoshimura, N. Shibata, M. Kawakami, S. Sakaguchi, Ligand design for
dual enantioselective control in Cu-catalyzed asymmetric conjugate addition
of R2Zn to cyclic enone, Tetrahedron, 68(17), 3512-3518 (2012). [invited paper] |
|
 |
|
|
| ・ |
| |